Answer:
Numerical value of
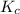 is 242
is 242
Step-by-step explanation:
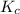 for the given reaction is represented as-
for the given reaction is represented as-
![K_(c)=([SO_(3)]^(2))/([SO_(2)]^(2)[O_(2)])](https://img.qammunity.org/2021/formulas/chemistry/college/b2p47wzx5abmj1jioc1kyfc77r0qb9cwdq.png)
Where species inside third bracket represent equilibrium concentrations.
Here,
![[SO_(3)]=0.66M](https://img.qammunity.org/2021/formulas/chemistry/college/e4qmwltu7xwq330fg6fgaj2517dum1lxaw.png) ,
,
![[SO_(2)]=0.10M](https://img.qammunity.org/2021/formulas/chemistry/college/melh1593qer3k9fy6lbmjesec53np2v3az.png) and
and
![[O_(2)]=0.18M](https://img.qammunity.org/2021/formulas/chemistry/college/4igtx1ekvxaw1qcnyu4ncb8i9jtfi26sy5.png)
Plug-in all the given values in the above equation-
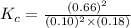
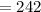
So, numerical value of
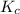 is 242
is 242