This is an incomplete question, here is a complete question.
The combustion of ethylene proceeds by the reaction
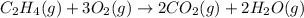
When the rate of disappearance of O2 is 0.42 Ms−1, the rate of disappearance of C₂H₄ is ________ Ms−1.
(a) 0.84 (b) 0.28 (c) 0.14 (d) 0.63 (e) 1.3
Answer : The rate of disappearance of C₂H₄ is, (c) 0.14 M/s
Explanation :
The general rate of reaction is,
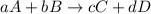
Rate of reaction : It is defined as the change in the concentration of any one of the reactants or products per unit time.
The expression for rate of reaction will be :
![\text{Rate of disappearance of A}=-(1)/(a)(d[A])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/l5nq5dm6c64x5qwmlag95leelb2etlzkr1.png)
![\text{Rate of disappearance of B}=-(1)/(b)(d[B])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/5l3cg3sto8meef7oycfaod1u3wvsuw9ub7.png)
![\text{Rate of formation of C}=+(1)/(c)(d[C])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/xw0wwcz1va7f3anj1gdbsxfi483mlyz3o1.png)
![\text{Rate of formation of D}=+(1)/(d)(d[D])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/wj1sd90jqqrsnw295fwngcqwmzos2a7f91.png)
![Rate=-(1)/(a)(d[A])/(dt)=-(1)/(b)(d[B])/(dt)=+(1)/(c)(d[C])/(dt)=+(1)/(d)(d[D])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/6qoojj15vu8vlh04zn3cm6tshrj90qpi6c.png)
From this we conclude that,
In the rate of reaction, A and B are the reactants and C and D are the products.
a, b, c and d are the stoichiometric coefficient of A, B, C and D respectively.
The negative sign along with the reactant terms is used simply to show that the concentration of the reactant is decreasing and positive sign along with the product terms is used simply to show that the concentration of the product is increasing.
The given rate of reaction is,
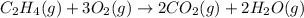
The expression for rate of reaction :
![\text{Rate of disappearance of }C_2H_4=-(d[C_2H_4])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/cpsc4c7bkzj55hmcirv8aw9s9xjxzjojr2.png)
![\text{Rate of disappearance of }O_2=-(1)/(3)(d[O_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/nwi8enyulwz4bqc2zpb1s5kp7xww3jtd2y.png)
![\text{Rate of formation of }CO_2=+(1)/(2)(d[CO_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/2gsatix3gk263m6c65lnd7i2lki1lfehcu.png)
![\text{Rate of formation of }H_2O=+(1)/(2)(d[H_2O])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/yl3n4mn4n2p8b7tq1q7o96f7a5n9l3b4me.png)
As we are given:
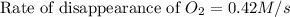
![-(d[C_2H_4])/(dt)=-(1)/(3)(d[O_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/5ygmlcby2qojmt43tkvm90wcj3qdfj2z9i.png)
![(d[C_2H_4])/(dt)=(1)/(3)(d[O_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/vkf4lo3pzuzg6j8ckg1yeymuy2cektl5rh.png)
![(d[C_2H_4])/(dt)=(1)/(3)* 0.42M/s](https://img.qammunity.org/2021/formulas/chemistry/college/6ykh5e8tcx7631f6bqsy8jer0t4aukq4it.png)
![(d[C_2H_4])/(dt)=0.14M/s](https://img.qammunity.org/2021/formulas/chemistry/college/har2g2gij50802e6vmrwwf8i9rjp8qqg70.png)
Thus, the rate of disappearance of C₂H₄ is, 0.14 M/s