Answer:
a) 2.333 kg of glycol was added in car's cooling system along with 5.0 kg of water.
b) The boiling point of coolant mixture is 103.91°C.
Step-by-step explanation:
a)
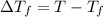
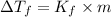
where,
 = freezing point of solution
= freezing point of solution
T = freezing point of solvent
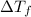 =depression in freezing point
=depression in freezing point
 = freezing point constant
= freezing point constant
m = molality =
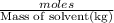
we have :
Mass of glycol = x
Mass of solvent = 5.0kg
Molality of the solution ,m=
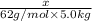
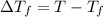
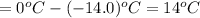
 =1.86°C/m ,
=1.86°C/m ,
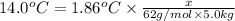
x = 2,333.33 g
2,333.33 = 2.3333 kg ( g = 0.001 kg)
2.333 kg of glycol was added in car's cooling system along with 5.0 kg of water.
b)
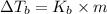
where,
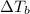 =elevation in boiling point =
=elevation in boiling point =
 = boiling point constant
= boiling point constant
m = molality
we have :
 =0.52°C/m ,
=0.52°C/m ,
Molality of the solution ,m=
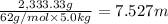
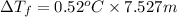
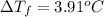
Boiling point of pure water = T = 100°C
Boiling point of solution =
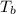
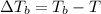
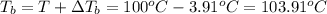
The boiling point of coolant mixture is 103.91°C.