Answer:
The heat capacity of a sample is 37.7 J/K.
Step-by-step explanation:
Given that,
Submerged temperature of tissue sample = 275 K
Mass of liquid nitrogen= 2 kg
Temperature = 70 K
Final temperature = 75 K
We need to calculate the heat
Using formula of heat
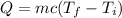
Put the value into the formula
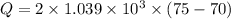
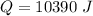
We need to calculate the heat capacity of a sample
Using formula of heat capacity
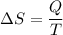
Put the value into the formula
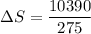
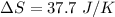
Hence, The heat capacity of a sample is 37.7 J/K.