Answer:
The pressure inside the syringe is 1.2 lbs/psi when the volume is 6 cubic centimeters.
Explanation:
We are given the following in the question:
When the plunger of a syringe is at a volume of 2 cubic centimeters, the pressure inside the syringe is 0.4 lbs/psi.
Boyle's Gas Law
- It states that the pressure of a gas is inversely proportional to volume of gas at a given temperature.
Thus, we can write:
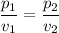
We have to find the pressure inside the syringe when the volume is 6 cubic centimeters.
Putting values, we get,
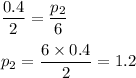
Thus, the pressure inside the syringe is 1.2 lbs/psi when the volume is 6 cubic centimeters.