Answer:
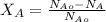
Nₐ₀-Nₐ = 1.33
Nₐ₀ = 2.5
Conversion X = 1.33/2.5 = 0.533
Step-by-step explanation:
A + 2B + 4C ⇒ 2X + 3Y
Given a stream containing 50% A, 25% B and 25% C, to get the limiting reactant, lets take a simple basis
Say stream is 10 moles, this give
A = 5moles
B = 2.5mole
C = 2.5moles
from the balanced equation above,
1mole of A ⇒ 4moles of C
∴ 5moles of A ⇒ (5x4)/1 ⇒ 20moles of C
also;
2mole of B ⇒ 4moles of C
∴ 2.5moles of B ⇒ (2.5x4)/2 ⇒ 5moles of C
so clearly from above reactant C is the limiting reactant.
Note: To get conversion of a process, we must use the limiting reactant. this is because ones it is used up, the reaction comes to an end
Formula to obtain conversion is:
Conversion = (Amount of A used up)/(Amount of A fed into the system)
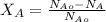
where, Nₐ₀-Nₐ = is the amount in moles of A used up
Nₐ₀ = amount in moles of A fed into the system
The next question is what mole of reactant C will give 0.1mole fraction of Y
Recall our basis = 10moles
from conservation of mass law, 10mole of product must come out which 0.1 moles fraction is Y
therefore amount Y in the product is = 0.1x10 = 1mole
if 3moles of Y ⇒ 4mole of C
∴ 1mole of Y ⇒ (1x4)/3 ⇒ 1.33moles of C
calculating the conversion of limiting reactant C that will give 0.1mole fraction of Y
Nₐ₀-Nₐ = 1.33
Nₐ₀ = 2.5
Conversion X = 1.33/2.5 = 0.533