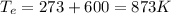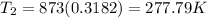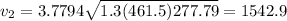Answer:
1542.9 m/s
Step-by-step explanation:
The table of thermodynamic properties of steam (Steam Table) is required to solve this question. Using steam table:
At p = 6000 kPa and T = 800°C, then entropy
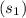 = 7.6554 kJ/(kg*K), internal energy
= 7.6554 kJ/(kg*K), internal energy
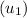 = 3641.2 kJ/kg, and
= 3641.2 kJ/kg, and
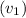 = 0.08159 m^3/kg. Thus:
= 0.08159 m^3/kg. Thus:
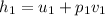 = 3641.2 + 6000*0.08159 = 3641.2 + 489.54 = 4130.74 kJ/kg
= 3641.2 + 6000*0.08159 = 3641.2 + 489.54 = 4130.74 kJ/kg
For condensation of steam to occur,
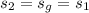
Thus,
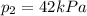 ,
,
 = 77°C,
= 77°C,
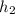 = 2638.8 kJ/kg
= 2638.8 kJ/kg
If we assume that steam is a perfect gas, we have:
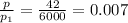 ,
,
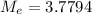 ,
,