Answer : The value of equilibrium constant (K) is, 0.004
Explanation :
First we have to calculate the concentration of
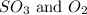
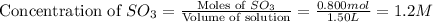
and,
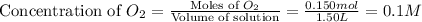
Now we have to calculate the value of equilibrium constant (K).
The given chemical reaction is:
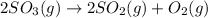
Initial conc. 1.2 0 0
At eqm. (1.2-2x) 2x x
As we are given:
Concentration of
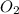 at equilibrium = x = 0.1 M
at equilibrium = x = 0.1 M
The expression for equilibrium constant is:
![K_c=([SO_2]^2[O_2])/([SO_3]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/wxbpqlmu8c70fbxiy64l4257agolm35co5.png)
Now put all the given values in this expression, we get:
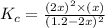
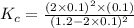
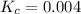
Thus, the value of equilibrium constant (K) is, 0.004