Answer:
The answer to the question is
The mass of NH3 (in grams) that must be used to produce 5.65 tons of HNO3 by the Ostwald process, assuming an 80.0 percent yield in each step in scientific notation is 3.46 × 10⁶ grams.
Step-by-step explanation:
The reaction for the Ostwald process is given by
4NH₃ + 5O₂ → 4NO + 6H₂O
2NO + O₂ ↔ 2NO₂
3NO₂ (g) + H₂O (l) → 2HNO₃ + NO
From the reactions of the Ostwald process, 4 moles of NH₃ are required to produce 2 moles of HNO₃
Therefore One mole of HNO₃ requires two moles of NH₃ for production at 100 % theoretical yield
Molar mass of NH₃ = 17.031 g/mol
Molar mass of HNO₃ = 63.01 g/mol
5.65 tons = 5.65 × 2000 lb/ton × 453.6 g/lb = 5125680 g
Number of moles of HNO₃ in 5125680 g sample of HNO₃
= (5125680 g)÷(63.01 g/mol) = 81347.09 moles of HNO₃
However the yield = 80.0% that is
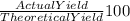 = 80 %
= 80 %
Therefore 80 % Theoretical yield = 81347.09 moles of HNO₃
Theoretical yield = 81347.09 moles ÷ 0.8 = 101683.86 moles
Then 101683.86 moles of HNO₃ will require 2 × 101683.86 moles or 203367.72 moles of NH₃
Mass of 203367.72 moles of NH₃ is given by
mass = number of moles × molar mass = 203367.72 moles × 17.031 g/mol = 3463555.63 grams
Mass of NH₃ required = 3463555.63 grams = 3.46 × 10⁶ grams in scientific notation.