The given question is incomplete. The complete question is as follows.
Sodium sulfate is slowly added to a solution containing 0.0500 M
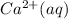 and 0.0390 M
and 0.0390 M
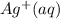 . What will be the concentration of
. What will be the concentration of
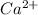 (aq) when
(aq) when
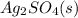 begins to precipitate? What percentage of the
begins to precipitate? What percentage of the
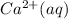 can be separated from the Ag(aq) by selective precipitation?
can be separated from the Ag(aq) by selective precipitation?
Step-by-step explanation:
The given reaction is as follows.
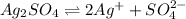
![[Ag^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/olsa8shvj9cd8zwjhfv1es86wljsrbwm1g.png) = 0.0390 M
= 0.0390 M
When
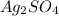 precipitates then expression for
precipitates then expression for
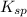 will be as follows.
will be as follows.
![K_(sp) = [Ag^(+)]^(2)[SO^(2-)_(4)]](https://img.qammunity.org/2021/formulas/chemistry/college/hukwdk6qy9l14jo7x0jm7l51a4nl9im4he.png)
![1.20 * 10^(-5) = (0.0390)^(2) * [SO^(2-)_(4)]](https://img.qammunity.org/2021/formulas/chemistry/college/b855qdmsmk85u88a8cxgrzbczt7s05ev2a.png)
![[SO^(2-)_(4)]](https://img.qammunity.org/2021/formulas/chemistry/college/5gtdn6mpjl8ax92nrrc99gvo8j7iqnwxix.png) = 0.00788 M
= 0.00788 M
Now, equation for dissociation of calcium sulfate is as follows.
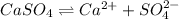
![K_(sp) = [Ca^(2+)][SO^(2-)_(4)]](https://img.qammunity.org/2021/formulas/chemistry/college/svxrubz2cop51nmsiuewlxar6q7euozyww.png)
![4.93 * 10^(-5) = [Ca^(2+)] * 0.00788](https://img.qammunity.org/2021/formulas/chemistry/college/e65tiq49glo9zbs9g4x1lqn29d8l19c468.png)
![[Ca^(2+)]](https://img.qammunity.org/2021/formulas/chemistry/college/8qboeq70y9plosxl9jadhlt6p32n466poh.png) = 0.00625 M
= 0.00625 M
Now, we will calculate the percentage of
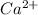 remaining in the solution as follows.
remaining in the solution as follows.
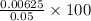
= 12.5%
And, the percentage of
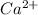 that can be separated is as follows.
that can be separated is as follows.
100 - 12.5
= 87.5%
Thus, we can conclude that 87.5% will be the concentration of
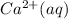 when
when
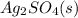 begins to precipitate.
begins to precipitate.