Answer:
Therefore the solubility of CaSO₄ in Na₂SO₄ is =907.12 g/L.
Step-by-step explanation:
CaSO₄ → Ca²⁺ + SO₄²⁻
Initially 0 0 0
Finally s s s
![K_(sp)=[Ca^(2+)][SO_4^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/h53ro6gppunn5lynrcocvoqc7wiwh56d49.png) ........(1)
........(1)
Na₂SO₄ → Na²⁺ + SO₄²⁻
Given, the concentration of Na₂SO₄ is 0.30 M .
Then [Na²⁺] = [SO₄²⁻] = 0.30
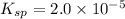
Putting the value of
 and [SO₄²⁻] in equation (1)
and [SO₄²⁻] in equation (1)
![2.0* 10^(-5)= [Ca^(2+)]0.30](https://img.qammunity.org/2021/formulas/chemistry/middle-school/8d1s4h2333rz1v4eouruu2minnls1d9msi.png)
![\Rightarrow [Ca^(2+)]=(2.0* 10^(-5))/(0.30)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/k6le8vy5pm4tjezuk6fe9govg3qmoxozgj.png)
= 6.67×10⁻⁵
The atomic mass of CaSO₄ is 136 g/mol
Therefore the solubility of CaSO₄ in Na₂SO₄ is = (6.67×10⁻⁵×136)g/L
=907.12 g/L