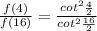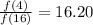Answer:
Ratio of number of alpha particles is 16.20
Step-by-step explanation:
In Rutherford Scattering, the fraction of incident beam particles scattered with angle θ and grater than θ is given by the relation :
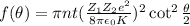
Here Z₁ is the atomic number of target, Z₂ is atomic number of incident particles, e is electronic charge, K is kinetic energy of incident particles, n is target particle density and t is thickness of the target foil.
According to the problem, number of alpha scattered to angles greater than 4° is given by the relation :
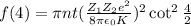 ....(1)
....(1)
Number of alpha scattered to angles greater than 16° is given by the relation :
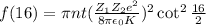 .....(2)
.....(2)
Ratio of equation (1) and (2) gives :