Answer:
Coffee at 38°C has higher kinetic energy than coffee at 34°C.
Step-by-step explanation:
- Kinetic energy is directly dependent upon the temperature of the object. As the temperature increases the velocity of the particle increases too. This higher velocity causes the average kinetic energy to increase.
- So in the cup of coffee with temperature 38°, C has higher kinetic energy than the one with 34°C.
- In the case of 0.25 L of tea, it has the highest temperature i.e. 43°C but the mass is less than coffee. According to relation, E= m
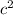 , the higher mass has higher energy. The particle with higher kinetic energy has higher mass because the fast-moving particle weighs more.
, the higher mass has higher energy. The particle with higher kinetic energy has higher mass because the fast-moving particle weighs more.