Answer:
- C. The rate of sublimation of the NH₄CI crystals is equal to the rate of solidification of the NH₄CI vapors.
Step-by-step explanation:
The question is garbled and the reactants and products do not match.
This is the correct question, assuming the products are correctly described:
"Consider the equilibrium system below.
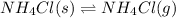
If the system is at dynamic equilibrium, which statement is true?
- A. Sublimation of the NH₄CI crystals stops.
- B. Solidification of the NH₄CI vapors stops.
- C. The rate of sublimation of the NH₄CI crystals is equal to the rate of solidification of the NH₄CI vapors.
- D. The rate of sublimation of the NH₄CI crystals is higher than the rate of solidification of the NH₄CI vapors"
Solution
The left side of the equation shows solid NH₄CI. The right side shows gas NH₄CI.
Thus, the equation represents the equilibrium between the formation of crystals (solid) and vapors (gas) of the compound NH₄CI.
The change from solid state to gas state is represented by the forward reaction (fom left to the right). It is named sublimation.
The change from vapors to crystals (named deposition) is represented by the reverse reaction (from right to left).
Dynamic equilibrium means that the molecules continually form vapors and cristals at the same speed. Thus, the final result is that each the total number of molecules of vapors and the total number of molecules of crystals do not change.
In conclusion, "The rate of sublimation of the NH₄CI crystals is equal to the rate of solidification of the NH₄CI vapors" (option C).