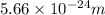Answer : The wavelength of the light emitted is,
Explanation :
The energy level of quantum particle in a one-dimensional box is given as:
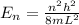
or,
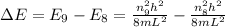
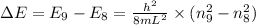
where,
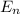 = change in energy
= change in energy
n = energy level
h = Planck's constant =
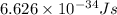
m = mass of electron =
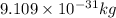
L = length of a one-dimensional box =
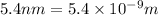
Now put all the given values in the above formula, we get:
![\Delta E=E_9-E_8=((6.626* 10^(-34)Js)^2)/(8* (9.109* 10^(-31)kg)* (5.4* 10^(-9)m)^2)* [(9)^2-(8)^2]](https://img.qammunity.org/2021/formulas/chemistry/college/dh5xxiipkfeeg608dewkzin7lw6h64uwgb.png)
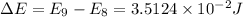
Now we have to calculate the wavelength of the light emitted.
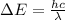
where,
h = Planck's constant =
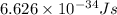
c = speed of light =
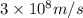
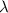 = wavelength of the light
= wavelength of the light
Now put all the given values in the above formula, we get:
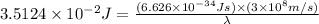
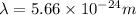
Thus, the wavelength of the light emitted is,