Answer:
![\large\boxed{\large\boxed{K_c=([Cu^(2+)])/([Ag^+]^2)}}](https://img.qammunity.org/2021/formulas/chemistry/college/2qimdlw7ixixbmcs55i7vlwk35wxeb0rgr.png)
Step-by-step explanation:
1. Chemical equilibrium equation:
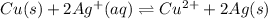
2. Species
In an equilibrium constant expression you do not include the solid substances; only gases and dissolved substances.
The symbol
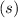 means solid, thus Cu(s) and Ag(s) shall not appear in your equilibrium constant expression.
means solid, thus Cu(s) and Ag(s) shall not appear in your equilibrium constant expression.
The symbol
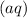 means in aqueous solution, thus the both
means in aqueous solution, thus the both
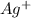 and
and
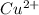 must appear in the equilibrium constant expression.
must appear in the equilibrium constant expression.
3. Equilibrium constant expression.
It is the quotient of the product of the concentrations of the species on the right hand side of the equilibrium equation, each raised to its corresponding coefficient, and the product of the concentrations of the species on the left hand side, each raised to its coresponding coefficient.
![K_c=([Cu^(2+)])/([Ag^+]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/me594iw1x1xbrgyqagargennvvwf1rujsg.png)