Answer:
Therefore the final volume reading in the graduated cylinder is 71.028 mL.
Step-by-step explanation:
Density: Density is determined by dividing the mass of the material with the volume of the material.
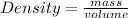
The S.I unit of mass is Kg
The C.G.S unit of mass is gram
The S.I unit of volume is m³
The C.G.S unit of volume is cm³
Therefore S.I unit of density is = Kg/m³
and C.G.S unit is g/cm³.
given that the mass of brass = 180 g
And the density of brass = 8.56g/mL
We know that,
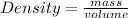
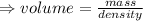
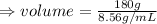
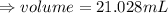
The graduated cylinder is containing 50.0 mL of water.
After the placing the brass into the Cylinder, then the volume become
=(volume of water+ volume of brass)
=(50.0+21.028)mL
=71.028 mL
Therefore the final volume reading in the graduated cylinder is 71.028 mL.