Answer: The rate law is
![rate=k[CH_3COOC_2H_5]^1[NaOH]^1](https://img.qammunity.org/2021/formulas/chemistry/college/ypzr3daceu87mm4qrsb5u2gsfd8l9bpkn8.png)
Step-by-step explanation:
Rate law says that rate of a reaction is directly proportional to the concentration of the reactants each raised to a stoichiometric coefficient determined experimentally called as order.
For the given reaction:
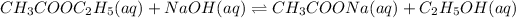
k= rate constant
Rate law:
![rate=k[CH_3COOC_2H_5]^x[NaOH]^y](https://img.qammunity.org/2021/formulas/chemistry/college/zfiatbctyedw43ny3ceikbtsl78aijvqvo.png)
For the given rate law:
y =1 = order with respect to

n = total order = 2
2= (x+y)
2= (x+1)
x= 1
Thus order with respect to
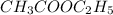 is 1 and rate law is :
is 1 and rate law is :
![rate=k[CH_3COOC_2H_5]^1[NaOH]^1](https://img.qammunity.org/2021/formulas/chemistry/college/ypzr3daceu87mm4qrsb5u2gsfd8l9bpkn8.png)