This is an incomplete question, here is a complete question.
If the initial concentration of NOBr is 0.0440 M, the concentration of NOBr after 9.0 seconds is ________.
The reaction
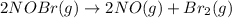
It is a second-order reaction with a rate constant of 0.80 M⁻¹s⁻¹ at 11 °C.
Answer : The concentration of after 9.0 seconds is, 0.00734 M
Explanation :
The integrated rate law equation for second order reaction follows:
![k=(1)/(t)\left ((1)/([A])-(1)/([A]_o)\right)](https://img.qammunity.org/2021/formulas/chemistry/college/a2lj3jeijlgwg6ljnsxcls8m69g41v1xla.png)
where,
k = rate constant = 0.80 M⁻¹s⁻¹
t = time taken = 142 second
[A] = concentration of substance after time 't' = ?
![[A]_o](https://img.qammunity.org/2021/formulas/chemistry/college/4pmjkus4n3cfntqcbgc1rjdz49krp7grr3.png) = Initial concentration = 0.0440 M
= Initial concentration = 0.0440 M
Putting values in above equation, we get:
![0.80M^(-1)s^(-1)=(1)/(142s)\left ((1)/([A])-(1)/((0.0440M))\right)](https://img.qammunity.org/2021/formulas/chemistry/high-school/x1ghkk0gmpi195hji34i2lut8p6reb19ri.png)
![[A]=0.00734M](https://img.qammunity.org/2021/formulas/chemistry/high-school/uvxrc3rkt39qcq1eau9wj8cz02k8orw1a3.png)
Hence, the concentration of after 9.0 seconds is, 0.00734 M