Answer:
11.61 is the pH of 10.0 mL of a solution containing 3.96 g of sodium stearate.
Step-by-step explanation:
Concentration of sodium stearate acid : c
Moles of sodium stearate =
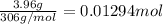
Volume of the solution = 10.0 mL = 0.010 L
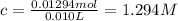
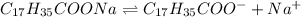
![[C_(17)H_(35)COO^-]=c=1.294 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/yrfojri1aluo796ci0rebzhgluxnzxolur.png)
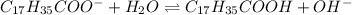
initially c
c 0 0
At equilibrium
(c-x) x x
Dissociation constant of an acid =
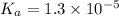
Expression of a dissociation constant of an acid is given by:
![K_a=([C_(17)H_(35)COOH][OH^-])/([C_(17)H_(35)COO^-])](https://img.qammunity.org/2021/formulas/chemistry/high-school/tdaflbytlu1q97aoyk3shkr5zpblle097e.png)
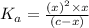
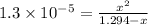
Solving for x;
x = 0.0041 M
![[OH^-]=0.0041 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/qgxyrmlq9pmlf7fyi21oe7jdvcfklur2bg.png)
The pOH of the solution:
![pOH=-\log[OH^-]=-\log[0.0041 M]=2.39](https://img.qammunity.org/2021/formulas/chemistry/high-school/enjtcqvmelvbit2w3ctenmtqsdj9mgkcdz.png)
pH = 14 -pOH
pH = 14 - 2.39 = 11.61
11.61 is the pH of 10.0 mL of a solution containing 3.96 g of sodium stearate.