Answer:
If pressure and number of moles are constant than the volume of a gas is directly proportional to its temperature.
Step-by-step explanation:
Ideal gas law is given as;
PV = nRT
Where;
P is the gass pressure
V is the gas volume
n is the number of moles of the gas
R is the gas constant
T is the gas temperature
If pressure and number of moles are constant, then the ideal gas equation will reduce to;
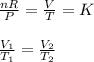
Thus, the volume of a gas is directly proportional to its Temperature