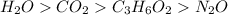Answer : The sample
 has greatest number of oxygen atoms.
has greatest number of oxygen atoms.
The order of number of oxygen atoms from greatest to least will be:
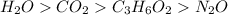
Explanation :
First we have to calculate the moles of
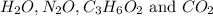
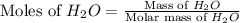
Molar mass of
 = 18 g/mol
= 18 g/mol
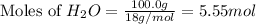
and,
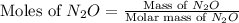
Molar mass of
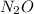 = 44 g/mol
= 44 g/mol
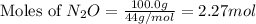
and,
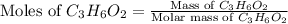
Molar mass of
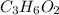 = 74 g/mol
= 74 g/mol
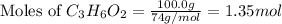
and,
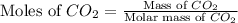
Molar mass of
 = 44 g/mol
= 44 g/mol
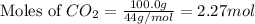
Now we have to calculate the number of oxygen atoms in
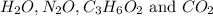
In
 :
:
As, 1 mole of
 contains
contains
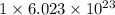 number of oxygen atoms
number of oxygen atoms
So, 5.55 mole of
 contains
contains
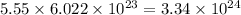 number of oxygen atoms
number of oxygen atoms
In
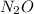 :
:
As, 1 mole of
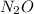 contains
contains
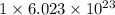 number of oxygen atoms
number of oxygen atoms
So, 2.27 mole of
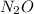 contains
contains
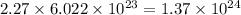 number of oxygen atoms
number of oxygen atoms
In
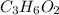 :
:
As, 1 mole of
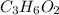 contains
contains
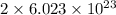 number of oxygen atoms
number of oxygen atoms
So, 1.35 mole of
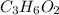 contains
contains
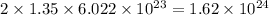 number of oxygen atoms
number of oxygen atoms
In
 :
:
As, 1 mole of
 contains
contains
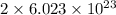 number of oxygen atoms
number of oxygen atoms
So, 2.27 mole of
 contains
contains
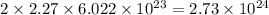 number of oxygen atoms
number of oxygen atoms
The order of number of oxygen atoms from greatest to least will be: