Answer : The volume of 6 M HCl needed is, 10 mL
Explanation :
Formula used :

where,
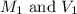 are the initial molarity and volume of diluted HCl.
are the initial molarity and volume of diluted HCl.
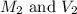 are the final molarity and volume of HCl.
are the final molarity and volume of HCl.
We are given:
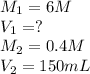
Now put all the given values in above equation, we get:
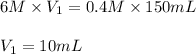
Hence, the volume of 6 M HCl needed is, 10 mL