Answer:
a)W=12.62 kJ/mol
b)W=12.59 kJ/mol
Step-by-step explanation:
At T = 100 °C the second and third virial coefficients are
B = -242.5 cm^3 mol^-1
C = 25200 cm^6 mo1^-2
Now according isothermal work of one mole methyl gas is
W=-
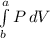
a=
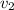
b=
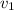
from virial equation
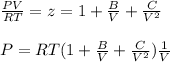
And
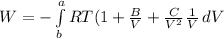
a=
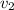
b=
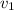
Now calculate V1 and V2 at given condition
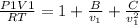
Substitute given values
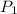 = 1 x 10^5 , T = 373.15 and given values of coefficients we get
= 1 x 10^5 , T = 373.15 and given values of coefficients we get
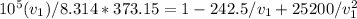
Solve for V1 by iterative or alternative cubic equation solver we get
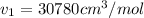
Similarly solve for state 2 at P2 = 50 bar we get
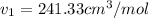
Now
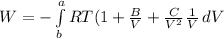
a=241.33
b=30780
After performing integration we get work done on the system is
W=12.62 kJ/mol
(b) for Z = 1 + B' P +C' P^2 = PV/RT by performing differential we get
dV=RT(-1/p^2+0+C')dP
Hence work done on the system is
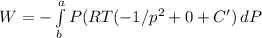
a=
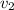
b=
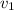
by substituting given limit and P = 1 bar , P2 = 50 bar and T = 373 K we get work
W=12.59 kJ/mol
The work by differ between a and b because the conversion of constant of virial coefficients are valid only for infinite series