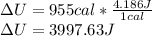Answer:
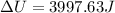
Step-by-step explanation:
According to the first law of thermodynamics:
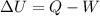
Here
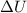 is the change in the internal energy of the system, Q is the supplied heat to the system and W is the work done by the system. The work done by the system is defined as:
is the change in the internal energy of the system, Q is the supplied heat to the system and W is the work done by the system. The work done by the system is defined as:
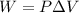
Where P is the presion and
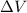 is the volume change. The volume remains constant, since the water are sealed in a rigid container:
is the volume change. The volume remains constant, since the water are sealed in a rigid container:
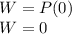
So, the change in internal energy of the water is:
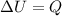
1 cal is equal to 4.186 J, so: