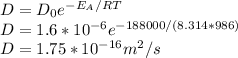Answer:
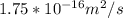
Step-by-step explanation:
Data given
Maximum diffusion coefficient,
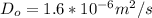
Activation Energy,
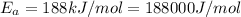
Temperature,T=713+273=986K
Universal gas constant, R=8.314J/mk
Since diffusion is the movement of particles from a higher concentration to a lower concentration, the coefficient of diffusion is expressed as
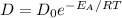
If we substittute valies we arrive at