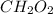Answer:
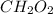
Step-by-step explanation:
What is the empirical formula of a substance that contains 2.64 g of C, 0.444 g of H, and 7.04 g of O?
First, find the number of mole of each element:
moles = mass/molar mass.
moles C = 2.64 g / 12.011 = 0.220
moles H = 0.444 g/ 1.008 = 0.440
moles = 7.04 g / 15.999 = 0.440
Divide each mole by the smallest mole
C = 0.220/0.220 = 1
H = 0.440/0.220 = 2
O = 0.440/0.220 = 2
Hence, the empirical formular is