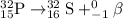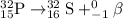 represents the balanced equation for the beta minus emission of phosphorus-32.
represents the balanced equation for the beta minus emission of phosphorus-32.
Option C
Step-by-step explanation:
Given choices:
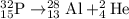
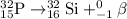
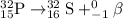
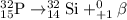
We need to find the balanced equation for the beta minus emission of phosphorus-32
Beta minus (β) is the decay process in which a neutron becomes a proton and releases beta particles, or better known as electrons. Means, atomic number gets increased by 1 (increasing one proton number).
So, from this concluding that in option C, Beta minus decay for phosphorus, it is replaced with sulfur core and increase in an atomic number (15 to 16). Hence, the balanced chemical equation for for the beta minus emission of phosphorus-32 is: