Answer: The equilibrium concentration of
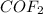 is 0.332 M
is 0.332 M
Step-by-step explanation:
We are given:
Initial concentration of
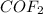 = 2.00 M
= 2.00 M
The given chemical equation follows:
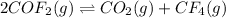
Initial: 2.00
At eqllm: 2.00-2x x x
The expression of
 for above equation follows:
for above equation follows:
![K_c=([CO_2][CF_4])/([COF_2]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/rldeuskdfeiwa3ex8ekjksq6x9ojp2wyoa.png)
We are given:

Putting values in above expression, we get:
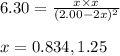
Neglecting the value of x = 1.25 because equilibrium concentration of the reactant will becomes negative, which is not possible
So, equilibrium concentration of
![COF_2=(2.00-2x)=[2.00-(2* 0.834)]=0.332M](https://img.qammunity.org/2021/formulas/chemistry/college/plp3osc3zysbwk29em3swdl2collsj9f4d.png)
Hence, the equilibrium concentration of
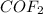 is 0.332 M
is 0.332 M