Answer:
The abundance of first isotope is 69.15 %
The abundance of second isotope is 30.85 %
Step-by-step explanation:
The formula for the calculation of the average atomic mass is:
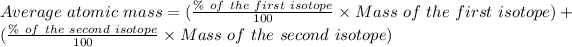
Given that:
Since the element has only 2 isotopes, so the let the percentage of first be x and the second is 100 -x.
For first isotope:
% = x %
Mass = 62.9296 u
For second isotope:
% = 100 - x
Mass = 64.9278 u
Given, Average Mass = 63.546 u
Thus,
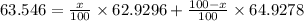
Solving for x, we get that:
x = 69.15 %
The abundance of first isotope is 69.15 %
The abundance of second isotope is 100 - 69.15 % = 30.85 %