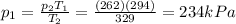1) 1.296 mol
2)
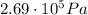
3) Yes
4) 234 kPa
Step-by-step explanation:
1)
In order to find the number of moles inside each tire, we can use the equation of state for an ideal gas:
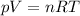
where:
p is the pressure of the gas
V its volume
n the number of moles
R the gas constant
T the absolute temperature
At the beginning, we have:
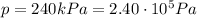
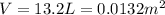
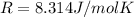
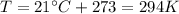
Therefore, the number of moles of nitrogen in each tire is:
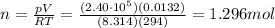
2)
The equation of state can be rewritten as
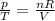
For the nitrogen gas inside the tires, the quantity nR/V remains constant, so we can write:
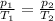
Where in this problem:
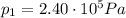 is the initial pressure
is the initial pressure
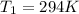 is the initial temperature
is the initial temperature
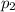 is the final pressure in Death Valley
is the final pressure in Death Valley
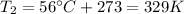 is the temperature in Death Valley
is the temperature in Death Valley
Solving for p2, we find the final pressure of the tires:
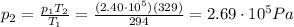
3)
As we have calculated in part 2, the pressure of the gas inside the tires when the car reaches the Death Valley will be
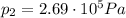
Which can be rewritten as
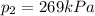
The text of the problem states that the tires will burst if the internal pressure reaches a value of
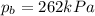
We observe that
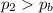
Which means that the internal pressure is larger than the breaking pressure: so, the tires will burst.
4)
Here we want the final pressure in the tires to be at most equal to the breaking pressure: so it must be
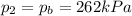
We can use again the equation used in part 2:
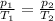
In order to find
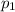 , the initial pressure at which the tires should be in order not to burst when the car arrives in the Death Valley.
, the initial pressure at which the tires should be in order not to burst when the car arrives in the Death Valley.
The temperatures are
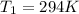 is the initial temperature
is the initial temperature
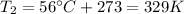 is the temperature in Death Valley
is the temperature in Death Valley
So the initial pressure must be