Answer:
Area = 201.1
Cicumference = 50.27
Your answers are correct
Explanation:
A = Area = ?
C = Circumference = ?
SInce both the "Area" and "Circumference" are unknown, we use the formula:
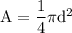
Remember that:
The diameter is the length of the line through the center
and
The radius is half the diameter
Given:
Diameter = 16
Now we substitute "16" for "d" into the formula and solve
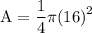
Calculate 16 to the power of 2 and get 256
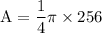
Multiply
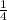 and 256 to get
and 256 to get
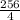
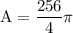
Divide 256 by 4 to get 64
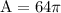
Multiply 64 and π to get 201.06192983
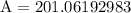
Round to the nearest tenth and get 201.1
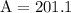
Next we need to find the "Circumference"
Formula for finding circumference is:
C = πd
Given:
Diameter = 16
Now we substitute "16" for "d" into the formula and solve
C = π(16)
Multiply 16 and π to get 50.265482457
C = 50.265482457
Round to the nearest hundreth and get 50.27
C = 50.27
So, your answers are correct.
The only mistake that could happen is whether you need to round to the nearest hundreth or tenth.