Answer:
(a) Rate at which
 is formed is 0.050 M/s
is formed is 0.050 M/s
(b) Rate at which
 is consumed is 0.0250 M/s.
is consumed is 0.0250 M/s.
Step-by-step explanation:
The given reaction is:-
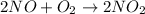
The expression for rate can be written as:-
![-(1)/(2)(d[NO])/(dt)=-(d[O_2])/(dt)=(1)/(2)(d[NO_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/86eqrk45iafe3vsm12mi4ln2pm1j5n2rws.png)
Given that:-
![(d[NO])/(dt)=-0.050\ M/s](https://img.qammunity.org/2021/formulas/chemistry/high-school/w7myxuoaprxwocffze7k6se3exz4l9gwys.png) (Negative sign shows consumption)
(Negative sign shows consumption)
![-(1)/(2)(d[NO])/(dt)=(1)/(2)(d[NO_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/k040nz2iokeo7qr8lhm33z6qb3404nmusy.png)
![-(d[NO])/(dt)=(d[NO_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/5gwj0v2ib0tdgilf1h5gb0rmxs50dp5bx4.png)
![-(-0.050\ M/s)=(d[NO_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/bk6l2nextw2u7dfdk0w66281s16k8fangc.png)
![(d[NO_2])/(dt)=0.050\ M/s](https://img.qammunity.org/2021/formulas/chemistry/high-school/evx8aqswtq633qwnqmp3kbfa6ph0c70qjc.png)
(a) Rate at which
 is formed is 0.050 M/s
is formed is 0.050 M/s
![-(1)/(2)(d[NO])/(dt)=-(d[O_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/8sqz7p6yojob2482orwcw0zw0bgh7kszr6.png)
![-(1)/(2)* -0.050\ M/s=-(d[O_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/wnckkttio3por11nda6wwpznef8vde1tcw.png)
![(d[O_2])/(dt)=0.0250\ M/s](https://img.qammunity.org/2021/formulas/chemistry/high-school/wd5tj5mexzshp972n5b0xi639f8eusnq6e.png)
(b) Rate at which
 is consumed is 0.0250 M/s.
is consumed is 0.0250 M/s.