Answer:
Plot ln K vs 1/T
(a) -0.5004; (b) 0.002 539 K⁻¹; (c) -197.1 K⁻¹; (d) 1.64 kJ/mol
Step-by-step explanation:
This is an example of the Arrhenius equation:
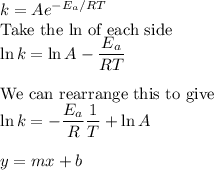
Thus, if we plot ln k vs 1/T, we should get a straight line with slope = -Eₐ/R and a y-intercept = lnA
Data:
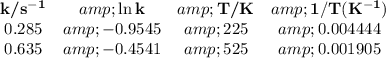
Calculations:
(a) Rise
Δy = y₂ - y₁ = -0.9545 - (-0.4541) = -0.9545 + 0.4541 = -0.5004
(b) Run
Δx = x₂ - x₁ = 0.004 444 - 0.001 905 = 0.002 539 K⁻¹
(c) Slope
Δy/Δx = -0.5004/0.002 539 K⁻¹ = -197.1 K⁻¹
(d) Activation energy
Slope = -Eₐ/R
Eₐ = -R × slope = -8.314 J·K⁻¹mol⁻¹ × (-197.1 K⁻¹) = 1638 J/mol = 1.64 kJ/mol