Position and momentum relation.
Step-by-step explanation:
- Heisenberg's uncertainty principle was given by Werner Karl Heisenberg in the year 1927.
- This principle gives the relation to the position of a particle and the momentum of a particle.
- The uncertainty principle states that the position of a particle and momentum of a particle cannot be measured at the same moment.
- This uncertainty is due to high precision.
The formula for uncertainty principle:
ΔxΔp ≥
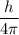
Here, Δx denotes uncertainiy in position and Δp denotes uncertainty in momentum.
- Smaller is the Δx larger is the Δp that means the more accurately uncertainty in position is measured there is less accuracy in measuring uncertainty in momentum.