Answer:
2. Increase; 4. (?)
Step-by-step explanation:
It looks as if you are finding the molar mass of a solute by measuring the freezing point depression of lauric acid.
The freezing point depression formula is
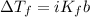
where
i = the van't Hoff factor of the solute
Kf = the molal freezing point depression constant of the solvent, and
b = the molal concentration of the solute
2. Effect of impure solute
If the impurity is insoluble in lauric acid, only part of the unknown will dissolve in the solvent. The observed freezing point depression will be too small. You will think there are fewer moles of solute.
moles = mass/molar mass
If you think the mass is correct, and the number of moles is too small, your calculated molar mass will be too high.
4. Effect of ionic solute
The problem won't arise with lauric acid as the solvent, because it is nonpolar and won't dissolve ionic solutes.
However, if you had a polar solvent like water, you would have twice as many particles in solution (i = 2). Your observed freezing point depression will be too large. You will think there are more moles of solute than are actually present, and your calculated molar mass will be too low.