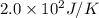Answer: The entropy change of the process is
Step-by-step explanation:
To calculate the entropy change for different phase at same temperature, we use the equation:
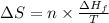
where,
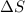 = Entropy change
= Entropy change
n = moles of acetone = 6.3 moles
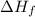 = enthalpy of fusion = 5.7 kJ/mol = 5700 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy of fusion = 5.7 kJ/mol = 5700 J/mol (Conversion factor: 1 kJ = 1000 J)
T = temperature of the system =
![-94.7^oC=[273-94.7]=178.3K](https://img.qammunity.org/2021/formulas/chemistry/college/jiw13hz46hw9fmdx3csgrztlddntish7ap.png)
Putting values in above equation, we get:
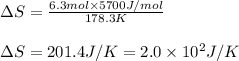
Hence, the entropy change of the process is