Answer:
Vapors
Step-by-step explanation:
We take into account that all the energy from the lightning has been transformed into steam.
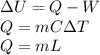
We calculate the amount of energy required by water to convert into steam.
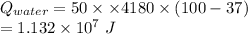
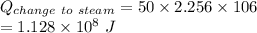
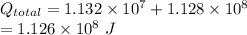
From the lightning we received
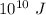 of energy, out of which
of energy, out of which
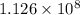 has been used to convert the water into steam.
has been used to convert the water into steam.
Energy left =
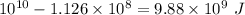
We use this energy to convert steam into vapors.
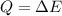
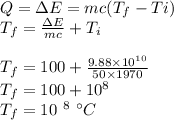
With this temperature, we can easily interpret that the vapors will be dissociated in hydrogen and oxygen particles.