This is an incomplete question, here is a complete question.
A weighed amount of sodium chloride is completely dissolved in a measured volume of 4.00 M ammonia solution at ice temperature, and carbon dioxide is bubbled in. Assume that sodium bicarbonate is formed util the limiting reagent is entirely used up. the solubility of sodium bicarbonate in water at ice temperature is 0.75 mol per liter. Also assume that all the sodium bicarbonate precipitated is collected and converted quantitatively to sodium carbonate.
Data to be used for calculating the results
-The mass of sodium chloride in (g) is 14.19
-The volume of ammonia solution in (mL) is 36.15
Calculate the following: What is the theoretical yield of sodium bicarbonate in grams?
Answer : The theoretical yield of sodium bicarbonate in grams is, 20.4 grams.
Explanation :
First we have to calculate the moles of NaCl and
 .
.
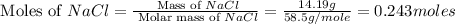
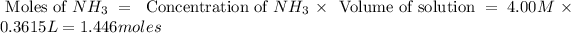
Now we have to calculate the limiting and excess reagent.
The balanced chemical reaction will be:
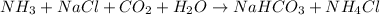
From the balanced reaction we conclude that
As, 1 mole of
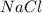 react with 1 mole of
react with 1 mole of

So, 0.243 mole of
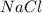 react with 0.243 mole of
react with 0.243 mole of

From this we conclude that,
 is an excess reagent because the given moles are greater than the required moles and
is an excess reagent because the given moles are greater than the required moles and
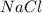 is a limiting reagent and it limits the formation of product.
is a limiting reagent and it limits the formation of product.
Now we have to calculate the moles of
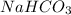
From the reaction, we conclude that
As, 1 mole of
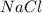 react to give 1 mole of
react to give 1 mole of
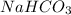
So, 0.243 moles of
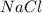 react to give 0.243 moles of
react to give 0.243 moles of
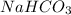
Now we have to calculate the mass of
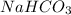
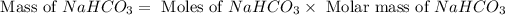
Molar mass of sodium bicarbonate = 84 g/mol
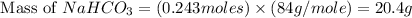
Thus, the theoretical yield of sodium bicarbonate in grams is, 20.4 grams.