Step-by-step explanation:
A compound or molecule which will have least dissociation and that is not able to given hydrogen ion easily upon dissociation will also have a low value of
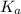 .
.
Dissociation of the given compounds or species will be as follows.
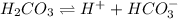
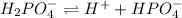
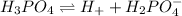
As, chemical formula of bicarbonate is
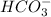 and due to the presence of a negative charge it is difficult to lose a positively charged hydrogen ion. This is because oppositely charged ions will be bonded by strong force of attraction.
and due to the presence of a negative charge it is difficult to lose a positively charged hydrogen ion. This is because oppositely charged ions will be bonded by strong force of attraction.
Hence, it will not easily lose a hydrogen ion due to which bicarbonate has the lowest
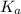 .
.
Thus, we can conclude that out of the given species bicarbonate has the lowest
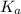 .
.