Step-by-step explanation:
Chemical equation for the given reaction is as follows.
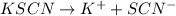
KSCN is present 35.0 ml of 0.06 M. Hence, we will calculate the total milimoles of
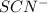 as follows.
as follows.
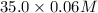
= 2.1 mmoles
Now, we will calculate the total volume in mixture as follows.
Total volume = (35 + 35 + 45) mL
= 115 ml
Hence, initial concentration of
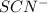 will be calculated as follows.
will be calculated as follows.
=
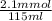
= 0.0182 M
Thus, we can conclude that the initial concentration of
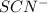 in the mixture is 0.0182 M.
in the mixture is 0.0182 M.