Answer : The percent yield of titanium (II) oxide is, 142.5 % and the impurities could have caused the percent yield to be so high.
Explanation : Given,
Mass of titanium(II) sulfide = 20.0 g
Molar mass of titanium(II) sulfide = 79.9 g/mole
Molar mass of titanium(II) oxide = 63.9 g/mole
First we have to calculate the moles of titanium(II) sulfide.
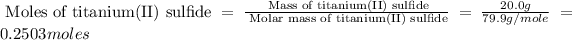
Now we have to calculate the moles of titanium(II) oxide.
The balanced chemical reaction is,
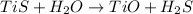
From the reaction, we conclude that
As, 1 mole of titanium(II) sulfide react to give 1 mole of titanium(II) oxide
So, 0.2503 mole of titanium(II) sulfide react to give 0.2503 mole of titanium(II) oxide
Now we have to calculate the mass of titanium(II) oxide.
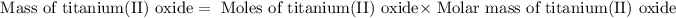
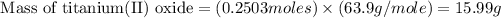
To calculate the percentage yield of titanium (II) oxide, we use the equation:
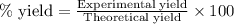
Experimental yield of titanium (II) oxide = 22.8 g
Theoretical yield of titanium (II) oxide = 15.99 g
Putting values in above equation, we get:
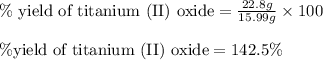
Hence, the percent yield of titanium (II) oxide is, 142.5 %
If the percent yields is greater than 100% that means the product of the reaction contains impurities which cause its mass to be greater than it actually.