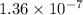Answer: The equilibrium constant for
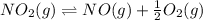 equation is
equation is
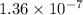
Step-by-step explanation:
The given chemical equation follows:
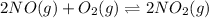
The value of equilibrium constant for the above equation is
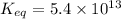
Calculating the equilibrium constant for the given equation:
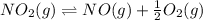
The value of equilibrium constant for the above equation will be:
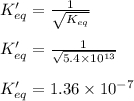
Hence, the equilibrium constant for
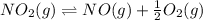 equation is
equation is