Answer:
The correct answer is option A.
Step-by-step explanation:
Step 1 : Slow
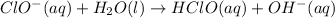
Step 2: Fast
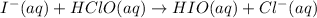
Step 3: Fast
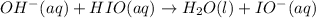
When there is a recation which is taking place in a more that single steps than the rate of the reaction is determined by the slowest step occurring in the reaction mechanism.
So, according to question step 1 is slow step which means that rate of the reaction will be :
![R=k[ClO^-][H_2O]](https://img.qammunity.org/2021/formulas/chemistry/college/7uxbi6m92n6x9p6u8w3sjuzqsy88n831cu.png)