Answer:
Step-by-step explanation:
Initial temperature of gas = pv / nR
= 1.5 x 29 / 2 x .082
= 265K
We can calculate the final temperature of gas as follows
T₂ = T₁
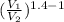
= 265 x 3°⁴
= 411K
Rise in temp = 411 - 265
= 146
increase in internal energy
=n
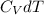
= 2x 5/2 R X 146
= 6059 J
Since there is rise in temperature , there will be rise in internal energy.