Answer:
0.6749 M is the concentration of B after 50 minutes.
Step-by-step explanation:
A → B
Half life of the reaction =
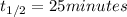
Rate constant of the reaction = k
For first order reaction, half life and half life are related by:
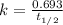
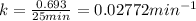
Initial concentration of A =
![[A]_o=0.900 M](https://img.qammunity.org/2021/formulas/chemistry/college/s2322e5h7t2z9zm8xyvs9rya2rgg4gqn6n.png)
Final concentration of A after 50 minutes =
![[A]=?](https://img.qammunity.org/2021/formulas/chemistry/college/oghjv7nqreoebvu50bk4s0gmrm7i7qo3q0.png)
t = 50 minute
![[A]=[A]_o* e^(-kt)](https://img.qammunity.org/2021/formulas/chemistry/college/eevx48o26qwcd7prplrrzkcmfv6xdtjpra.png)
![[A]=0.900 M* e^{-0.02772 min^(-1)* 50 minutes}](https://img.qammunity.org/2021/formulas/chemistry/college/mc9jdbm1rug8gjz2ih39dxvv1el2q1nvgs.png)
[A] = 0.2251 M
The concentration of A after 50 minutes = 0.2251 M
The concentration of B after 50 minutes = 0.900 M - 0.2251 M = 0.6749 M
0.6749 M is the concentration of B after 50 minutes.