Answer:
0.02405 g/L is the solubility of argon in water at 25 °C.
Step-by-step explanation:
Henry's law states that the amount of gas dissolved or molar solubility of gas is directly proportional to the partial pressure of the liquid.
To calculate the molar solubility, we use the equation given by Henry's law, which is:
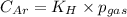
where,
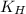 = Henry's constant =
= Henry's constant =
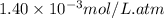
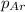 = partial pressure of carbonated drink = 0.51atm
= partial pressure of carbonated drink = 0.51atm
Putting values in above equation, we get:
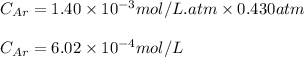
Molar mass of argon = 39.95 g/mol
Solubility of the argon gas :
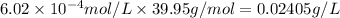
0.02405 g/L is the solubility of argon in water at 25 °C.