Answer:
The answers to the question are
(a) The the mg/L of H₃PO₄is 1462.05 g
(b) The molarity of H₃PO₄ in the solution is 14.92 mol/L
(c) The normality of H₃PO₄ in the solution 44.85 eq/L
Step-by-step explanation:
(a) The commercially available H₃PO₄ has a concentration of 85.5 percent by weight, therefore
The mass of H₃PO₄ is found by
Density of water = 1.71 g/ml
mass of one liter of acid solution = 1.71 ×1000 = 1710 g
Therefore 85.5 % by weight of H₃PO₄ =1710×85.5/100 = 1462.05 g
Therefore we have 1462.05 g/L of H₃PO₄
Molar mass of H₃PO₄ = 97.994 g/mol
(b)Therefore the number of moles in 1462.05 g = 14.92 moles and the molarity = 14.92 mol/L
(c) The Normality =
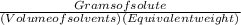 = 1465.05÷(1×97.994/3) = 44.85 eq/L
= 1465.05÷(1×97.994/3) = 44.85 eq/L