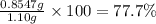Answer:
Mass percentage of glucose : 22.3%
Mass percentage of sucrose:77.7%
Step-by-step explanation:
Mass of glucose in sample =
Mass of sucrose in sample = y
Mass of sample = 1.10 g
x + y = 1.10 g ..[1]
Osmotic pressure of the solution =
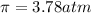
Volume of the solution = 25.0 mL = 0.025L ( 1 mL = 0.001 L)
Temperature of the solution =T = 298 K
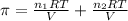
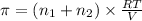
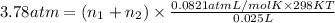
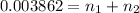
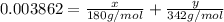
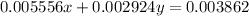 ..[2]
..[2]
Solving [1] and [2] we get :
x = 0.2453 g
y = 0.8547 g
Mass percentage of glucose :
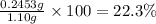
Mass percentage of sucrose: