Answer : The mass of benzoin is, 8.58 grams.
Explanation :
First we have to calculate the moles of benzil.
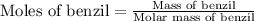
Molar mass of benzil = 210.23 g/mol
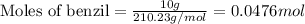
Now we have to calculate the moles of benzoin.
The balanced chemical reaction is:
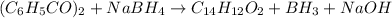
From the balanced chemical reaction we conclude that,
As, 1 mole of benzil react to give 1 mole of benzoin
So, 0.0476 mole of benzil react to give 0.0476 mole of benzoin
Now we have to calculate the mass of benzoin.
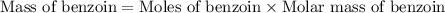
Molar mass of benzoin = 212.24 g/mol
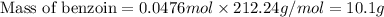
Theoretical yield of benzoin = 10.1 g
Now we have to calculate the experimental mass of benzoin.
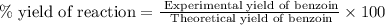
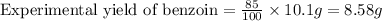
Therefore, the mass of benzoin is, 8.58 grams.